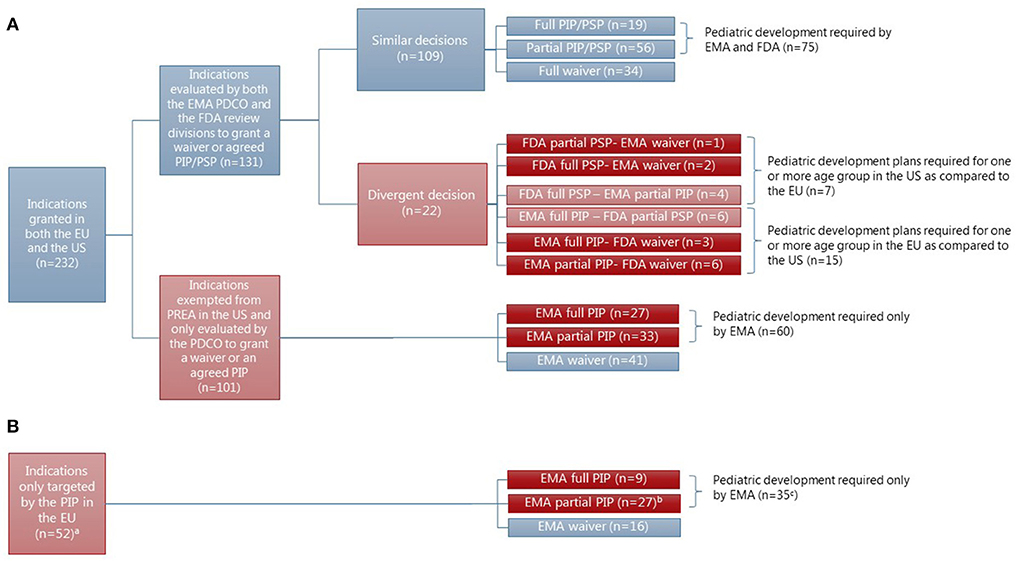
Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram
FDA / EMA Common Commentary on Submitting an initial Pediatric Study Plan (iPSP) and Paediatric Investigation Plan (PIP) for the

Timelines for PIP and PSP process. PSP review slide provided from the FDA. | Download Scientific Diagram

CurePSP on Twitter: "Because it is a pure tauopathy and is considered an orphan disease by the FDA, PSP is becoming a target for pharmaceutical companies looking to develop treatment, which may
FDA / EMA Common Commentary on Submitting an initial Pediatric Study Plan (iPSP) and Paediatric Investigation Plan (PIP) for the

FDA Advisory Meeting Clinical Pharmacology Review Utilizes a Quantitative Systems Pharmacology (QSP) Model: A Watershed Moment? – topic of research paper in Clinical medicine. Download scholarly article PDF and read for free